BREAKING: Ketryx Raises $39M Series B from Transformation & Lightspeed to Scale AI in MedTech
$57M Total Funding
Listen to full episode in Sourcery ^
Ketryx Raises $39M Series B
Closed in just 14 days, Ketryx has raised a $39M Series B, bringing its total funding to $57M, in a round led by Transformation Capital (Vinay Shah) with participation from Lightspeed Venture Partners (Guru Chahal), MIT’s E14 Fund, Ubiquity Ventures, 53 Stations, and notable angels. Cementing Ketryx as the leader in AI compliance infrastructure layer for MedTech and beyond.
→ Listen on X & in Sourcery
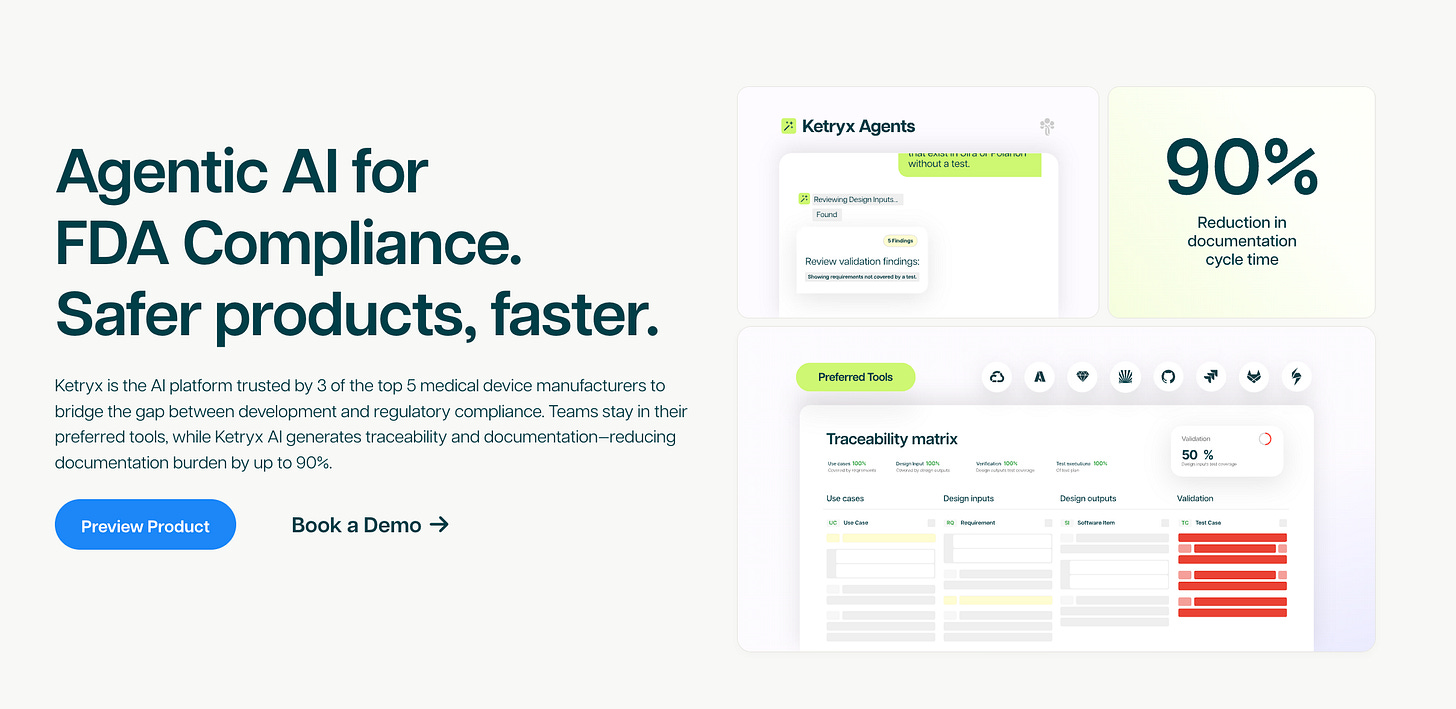
Trusted already by 3 of the top 5 MedTech companies, Ketryx enables highly regulated teams to move from year-long software release cycles to daily updates, without sacrificing safety. The AI-native platform uses enforces compliance across tools like Jira and GitHub, automates validation, and generates regulatory-grade documentation resulting in a 90% reduction in validation/documentation overhead.
Ketryx Founder & CEO Erez Kaminski joins Sourcery to explain why validation is the biggest bottleneck in healthcare innovation, how Ketryx already powers 2–3% of FDA AI approvals, & why top health tech investors believe Ketryx is the future of AI in safety-critical systems.
We’re testing out solely sharing this episode in Sourcery & on X, to watch/listen use the embedded episode at the top of the newsletter.
Brought to you by:
Brex—The modern finance platform, combining the world’s smartest corporate card with integrated expense management, banking, bill pay, and travel.
As a Sourcery subscriber you get: 75,000 points after spending $3,000 on Brex card(s), white-glove onboarding, $5,000 in AWS credits, $2,500 in OpenAI credits, & access to $180k+ in SaaS discounts. On top of $500 toward Brex travel, $300 in cashback, plus exclusive perks (like billboards..) visit → brex.com/sourcery
Turing—Turing delivers top-tier talent, data, and tools to help AI labs improve model performance, and enable enterprises to turn those models into powerful, production-ready systems. Visit: turing.com/sourcery
Kalshi—The largest prediction market & the only legal platform in the US where people can trade directly on the outcomes of future events (sports, politics, weather, AI, etc).
Ketryx Raises $39M to Accelerate AI in MedTech
The Announcement
Ketryx, the AI platform that automates compliance and validation for safety-critical software, has raised a $39 million Series B led by Transformation Capital, with participation from Lightspeed Venture Partners, E14 Fund, Ubiquity Ventures, 53 Stations, and several leading angels. The round brings Ketryx’s total funding to $57 million.
The raise came together quickly.. just 14 days from first serious conversations to a signed term sheet. Underscoring the urgency of the problem and the conviction of investors. Transformation Capital’s deep experience in healthcare IT and scaling safety-critical infrastructure made them a natural lead.
Ketryx already counts three of the top five global MedTech companies as customers. Products built on Ketryx accounted for 2–3% of FDA software approvals in 2024, validating the company’s impact in a short time.
The State of MedTech
MedTech has moved far beyond scalpels and basic devices. Today, the sector is defined by cloud-connected systems, surgical robots, and AI-driven diagnostics. But the regulatory and validation infrastructure hasn’t kept up.
Consumer software teams ship updates daily or hourly.
In life sciences and MedTech, equivalent-scale companies often release software only once every year or two.
That gap matters. Delayed updates can slow innovation and limit patient access to treatments, while also leaving products exposed to vulnerabilities and quality issues.
Why Regulation Isn’t the Problem
A common refrain in the industry is that regulation stifles innovation. Kaminski argues the opposite:
The FDA already allows for rapid AI updates in high-risk medical devices, even as frequently as hourly, provided the right infrastructure and evidence are in place.
The bottleneck isn’t the regulators—it’s the manual, document-centric compliance processes many companies still use, often built on outdated systems from the 1980s and 1990s.
In other words: regulation sets the rules of the road, but companies are still driving with 30-year-old maps.
Why This Solution Matters
Ketryx provides the infrastructure layer for regulated AI:
Integrations with Jira, GitHub, AWS, and other enterprise systems
AI agents that enforce compliance automatically, create guardrails, and generate regulatory-grade documentation
Zero-lag compliance, meaning companies can be release-ready at any moment without slowing engineering
Ketryx even uses its own platform to validate itself—a first in the industry—and has gone through external audits to meet medical device standards.
The product doesn’t just make compliance faster; it enables the safe adoption of AI in highly regulated domains like MedTech, pharma, aerospace, and nuclear.
How Much Time & Money Ketryx Saves
Validation is often the single largest bottleneck in bringing medical innovations to market, consuming 30–40% of project costs. By automating this work, Ketryx delivers:
90% reduction in documentation time
Shift from annual releases to biweekly, or even daily, release cycles
Closure of 3–5% traceability gaps, improving both patient safety and audit readiness
The downstream effect is enormous: faster fixes to safety issues, faster delivery of innovations, and lower costs for companies and patients alike.
Ketryx estimates that its customers already touch 25 million patients each year, and the company has set a goal of reaching 100 million patients by 2030.
With its Series B, Ketryx is positioned to become the default compliance infrastructure for AI in safety-critical systems. By reframing validation as a problem of infrastructure and automation, rather than paperwork and regulation, the company is helping MedTech move at the pace of modern software—without compromising on safety.
The Team Behind Ketryx
Ketryx has scaled rapidly from a handful of employees at its founding to nearly 100 team members across Boston/Cambridge and Vienna, Austria. The company’s DNA combines world-class engineering talent with deep regulatory and quality expertise—exactly the cross-disciplinary mix required to modernize safety-critical software.
Erez Kaminski, Founder & CEO: Former head of AI at Amgen’s medical device group, special assistant to Stephen Wolfram at Wolfram Research, and MIT LGO alumnus (read Erez’s announcement here).
Global Footprint: Roughly 40 developers in Vienna, with the rest of the team based in Cambridge, MA.
Hiring Strategy: The company actively recruits from MIT’s Leaders for Global Operations (LGO) program, where Erez himself studied. Ketryx is now one of the largest employers of LGO alumni, drawing talent with both technical and business expertise.
Engineering Culture: Led by co-founder & CTO Jan Pöschko, the Vienna team includes prolific developers like Patrick Ecker (ReScript), Anton Ryder, and alumni from Bitpanda. Their focus: building the infrastructure to validate safety-critical AI systems.
Quality & Regulatory Leadership: Early partners like Paul Jones, who helped write key U.S. software regulations, bring unmatched credibility to the team.
Mission-Driven Talent: Many team members are drawn to the personal story behind Ketryx—Erez’s mother receiving a cochlear implant—which underscores the company’s mission to improve patient outcomes through technology.
With its Series B, Ketryx is continuing to hire across engineering, AI, design, quality, and go-to-market functions, building a culture that blends startup velocity with the rigor required for regulated industries
TLDR: After just 2 years, 3 of the top 5 MedTech companies already rely on Ketryx.
Regulation Isn’t the Problem: Today, the constant refrain is that regulation stifles innovation. This isn’t true in the life sciences. The problem is outdated compliance practices and ancient systems companies use (from the 80s/90s)
Validation is THE giant problem: Because we want to keep people safe, we need high validation requirements. The consequence is that releasing software for safety-critical applications is painfully slow (years) and expensive (~30-40% of total project costs). Companies are bogged down in documentation, resulting in year-long release cycles. The complexity of validation is also what hinders teams from adopting AI today.
The Solution: Ketryx uses AI to enforce compliance across developer tools, create guardrails for AI, and provide validated agents to speed workflows. We eliminate 90% of the delays caused by manual work and provide teams a structured framework for introducing AI into their validated SDLC.
Real traction: As I mentioned, 3 of the top 5 MedTech companies are on Ketryx, along with some other notable giants. In 2024, submissions for products built on Ketryx accounted for over 2% of FDA software approvals.
Brought to you by Brex:
Brex is the intelligent finance platform: cards, expenses, travel, bill pay, banking—wrapped into a high-performance stack. Built for scale. Trusted by teams that move fast.
Spend smarter. Move faster. Sourcery subscribers get: 75,000 points after spending $3,000 on Brex card(s). Plus, white-glove onboarding, $5,000 in AWS credits, $2,500 in OpenAI credits, and access to $180k+ in SaaS discounts. On top of $500 toward Brex travel, $300 in cashback, plus exclusive perks (like billboards..) visit → brex.com/sourcery